Can You Put Salt on Top of Snow
The following essay is reprinted with permission from ![]() The Conversation, an online publication covering the latest research.
The Conversation, an online publication covering the latest research.
Brrr… it's cold out there! Children are flocking to the boob tube in hopes of hearing in that location will be a snowfall day; the bread and milk aisles at grocery stores are empty because of an impending snowfall storm; and utility trucks are out spraying common salt or salt h2o on the roads.
We all know why the first ii happen—kids are excited for a day off of schoolhouse filled with hot chocolate and snowmen. Adults are stocking up on necessities. But what'due south up with those trucks?
They're working to protect drivers from slippery conditions past spraying stone salt or a solution of salt water to preclude ice germination. This table salt is very similar to the salt you have on your dinner table—it's the same sodium chloride, NaCl. There are some proprietary mixtures that contain other salts—such as potassium chloride (KCl) and magnesium chloride (MgCl)—merely they're non equally commonly used.
Road salt isn't equally pure as what you utilise on your food; information technology has a brownish grey color, mostly due to mineral contamination. Subjecting the environment to this common salt via runoff can accept some unintended consequences including negative effects on plants, aquatic animals and wetlands.
Just it'southward a cheap and effective fashion to protect roads from ice due to a elementary scientific principle: freezing point depression of solutions. The freezing point of pure h2o, the temperature at which it becomes ice, is 32 degrees Fahrenheit. And then if there'south snow, sleet or freezing rain and the basis is 32 F or colder, solid ice will grade on streets and sidewalks.
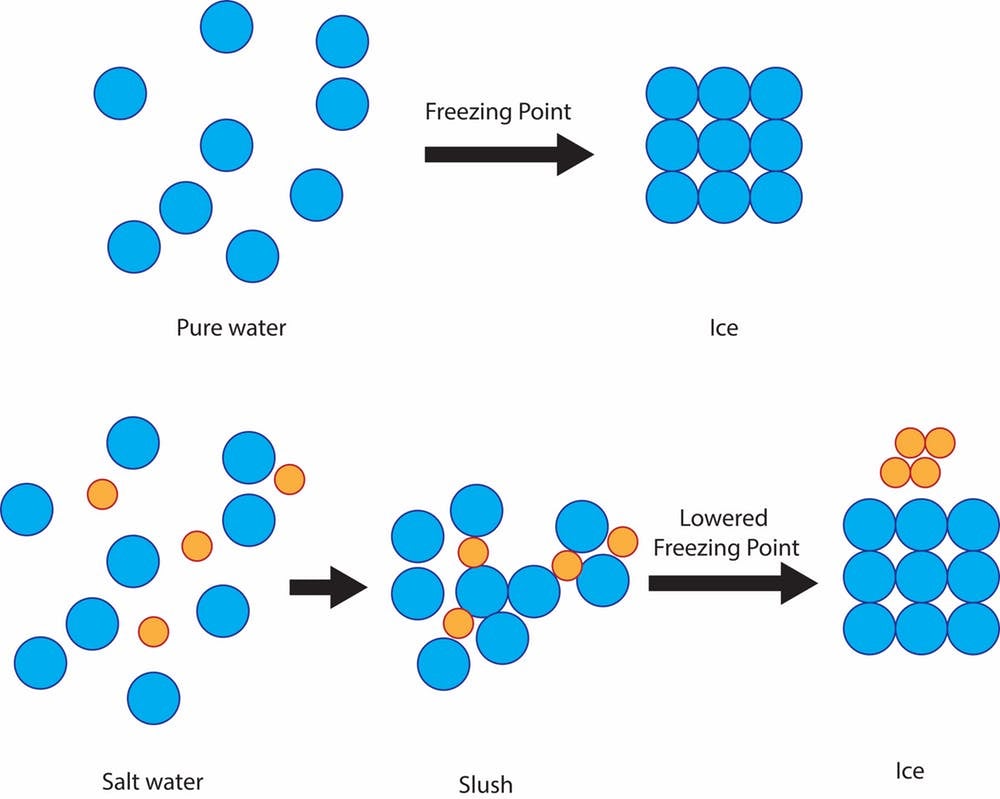
If the h2o is mixed with salt, though, the freezing temperature of the solution is lower than 32 F. The salt impedes the power of the h2o molecules to grade solid water ice crystals. The degree of freezing point depression depends on how salty the solution is.
It'due south important to note that the salt must be in a solution with liquid h2o in order for this principle to be obeyed. That's why many cities spray a salt solution earlier whatever water ice forms.
Salt that's dumped on superlative of ice relies on the dominicus or the friction of car tires driving over it to initially melt the ice to a slush that can mix with the salt and then won't refreeze. Pre-treating with solid table salt relies on the warmer road surface to initially melt any snow or freezing pelting so that it tin properly mix with the salt. This is also why pre-treatment of bridges—which are colder than other roads—does not typically work, and why yous see "bridge freezes before route" signs.
These table salt solutions decrease the freezing temperature of water to around fifteen F. So, unfortunately for folks facing truly frigid temps, treating with table salt won't get rid of ice on their roads.
An culling strategy used at these lower temperatures is putting sand on the ice. Sand doesn't change the melting temperature, it only provides a crude surface for your tires to preclude slipping and sliding.
The science of freezing signal depression can be applied to any solution, and many research groups have focused on developing alternatives with fewer negative environmental consequences. They include additives such every bit molasses and beet juice. And so maybe you lot can look forrad to cleaning not simply white salt off the bottom of your jeans after a winter walk, simply pink table salt as well.
This article was originally published on The Conversation. Read the original article.
Source: https://www.scientificamerican.com/article/salt-doesnt-melt-ice-heres-how-it-makes-winter-streets-safer/
0 Response to "Can You Put Salt on Top of Snow"
Post a Comment